Bis Tris Buffer Recipe : The Ultimate Guide to Creating Powerful Solutions
The Bis-Tris buffer recipe requires mixing equal parts of Bis-Tris propane and Tris base. This buffer is commonly used in biochemistry and molecular biology for controlling pH levels in protein assays and electrophoresis applications.
Bis-Tris buffer is highly effective in maintaining a stable pH in the range of 5. 8 to 7. 2, making it suitable for a wide range of experimental conditions. Its zwitterionic nature also makes it ideal for stabilizing proteins during analysis, ensuring accurate results.
Additionally, the buffer is compatible with metal ions and chelating agents, allowing for versatile use in various research settings. Not only does the Bis-Tris buffer provide consistent and reliable pH control, but it also minimizes buffer interactions with experimental samples, reducing potential interference with the desired biochemical reactions. As a crucial component in biochemical research, understanding how to prepare and utilize Bis-Tris buffer is essential for obtaining accurate and reproducible results.

Credit: www.yumpu.com
What Is A Bis Tris Buffer?
A Bis Tris buffer is a zwitterionic buffer with a pH range of 5.8 to 7.2, primarily used in electrophoresis and protein applications. It is stable at various temperatures and compatible with many enzyme activities. Due to its low ionic strength, Bis Tris is ideal for use in separating small proteins. This buffer is of importance in maintaining the desired pH levels essential for a variety of biological assays and experiments.
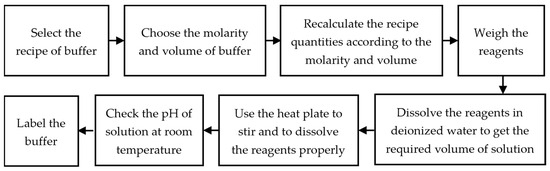
Credit: www.mdpi.com
Key Components
Bis Tris Buffer Recipe: The key components of this buffer include Bis and Tris as the buffering agents. Bis, also known as bis-tris, is a zwitterionic organic compound that maintains a stable pH in the buffer. Tris, or tris–hydroxymethylaminomethane, acts as a buffering agent with its neutralizing properties. It helps to maintain the stability of the pH level in the buffer solution.
Preparation And Recipe
Bis Tris buffer is commonly used in the laboratory for a variety of applications. Here is a simple recipe for preparing Bis Tris buffer:
Ingredients:
- Bis Tris: This is the main component of the buffer solution.
- Phosphoric acid: Used to adjust the pH of the buffer.
- Sodium hydroxide: Also used for pH adjustment.
- Distilled water: Used as the solvent for the buffer.
Step-by-step Instructions:
- Determine the desired final volume of the buffer solution.
- Calculate the amount of Bis Tris needed based on the concentration desired.
- Measure and add the appropriate amount of Bis Tris to a clean glass beaker.
- Add a small amount of distilled water to dissolve the Bis Tris completely.
- Slowly add phosphoric acid or sodium hydroxide to adjust the pH, using pH paper or a pH meter to monitor the pH level.
- Once the pH is adjusted, add distilled water to reach the desired final volume, while continuously stirring the solution.
- Transfer the buffer solution to a clean storage container and label it appropriately.
Applications And Uses
Bis Tris buffer is commonly used in electrophoresis applications. It provides stable pH conditions required for accurate separation of molecules. The buffer’s low conductivity allows for efficient electrophoretic mobility of charged molecules, ensuring accurate separation during the analysis. Whether you are analyzing DNA, RNA, or proteins, Bis Tris buffer can provide reliable results.
Bis Tris buffer is also effective in protein purification processes. Its ability to maintain stable pH levels is crucial for maintaining the structural integrity of proteins. Proteins can be easily eluted and purified in Bis Tris buffer conditions, allowing for successful downstream applications such as protein structure determination or functional studies.
Bis Tris buffer is widely used in enzyme activity assays. It provides optimal pH conditions required for enzyme-substrate interaction and accurate measurement of enzyme activity. The buffer’s ability to maintain a constant pH level ensures reliable and reproducible assay results. Bis Tris buffer has been proven to be compatible with a wide range of enzymes, making it a popular choice for various enzymatic assays.
Tips And Troubleshooting
In order to prepare a Bis Tris Buffer solution, it is important to be aware of some helpful tips and troubleshooting for successful results. When storing the solution, make sure to keep it at a temperature between 2 to 8 degrees Celsius. This will maintain the stability and effectiveness of the buffer solution. pH adjustment is crucial when using the buffer, as the optimal pH range is between 6.8 to 7.2. To ensure accuracy, use a pH meter or test strips to confirm the pH level before using the buffer.
There are a few common issues that might arise when working with a Bis Tris Buffer. One issue is precipitation, which can occur when the buffer is not dissolved properly. To avoid this, stir the solution gently and thoroughly until all the components are fully dissolved. Another issue can be the buffer becoming contaminated. It is important to handle the buffer with clean and sterile equipment to prevent contamination. Lastly, make sure to use the correct concentration of the buffer as specified in the recipe or protocol you are following.

Credit: www.fishersci.com
Frequently Asked Questions Of Bis Tris Buffer Recipe
What Is The Bis-tris Buffer System?
The Bis-Tris buffer system is a commonly used buffer for protein electrophoresis and enzyme assays. It maintains a stable pH range of 5. 8 to 7. 2 and helps reduce changes in pH caused by chemical reactions. Its ability to stabilize proteins makes it ideal for use in biomedical research and molecular biology techniques.
Is Bis-tris The Same As Tris-hcl?
Bis-Tris and Tris-HCl are different buffer systems used in biochemistry and have distinct properties for specific applications. Bis-Tris is a zwitterionic buffer while Tris-HCl is a salt of Tris.
How To Make A Tris Buffer?
To make a Tris buffer, dissolve Tris base in water, adjust pH with HCl, and dilute to desired volume.
What Is The Concentration Of Bis-tris Propane Buffer?
The concentration of bis-tris propane buffer is determined by the specific experiment or application. It can range from 10 mM to 200 mM, depending on the desired pH and buffer capacity.
Conclusion
The Bis Tris buffer recipe provides a reliable solution for maintaining optimal pH levels in various biological experiments. By following this recipe, scientists and researchers can ensure accurate and reproducible results. With its simple ingredients and straightforward preparation, the Bis Tris buffer is an essential tool in biochemical and molecular biology studies.
Start using this reliable buffer recipe today and enhance the accuracy of your experiments.